We are developing odiparcil for the treatment of several subtypes of MPS, a group of rare and progressive genetic disorders which manifest early in life
Odiparcil, an orally-available small molecule that can potentially decrease the lysosomal accumulation of chondroitin sulfate (CS) and dermatan sulfate (DS) in patients with certain MPS subtypes (MPS I, II, IVa, VI and VII).
Odiparcil changes the way DS and CS are synthesized, thereby facilitating the production of soluble DS and CS glycosaminoglycans (GAGs), which can be excreted in the urine, rather than accumulating in cells.
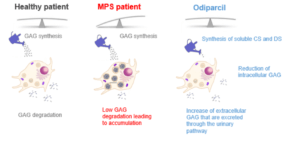
We believe odiparcil’s mechanism of action is relevant to a number of tissues in which GAGs accumulate and which are poorly addressed with limited efficacy by the current standard of care, which is enzyme replacement therapy (ERT). Unlike ERT, odiparcil is a small molecule that is well distributed in the body, even in tissues that are poorly vascularized or protected by a barrier such as the eyes.
In the recent iMProveS trial odiparcil demonstrated a dose of dependent increase of GAGs excretion via urine in MPS VI patients. At the same time, patients treated with odiparcil experienced improvements in organs such as the heart, lungs and eyes. For more information on the headline results of the iMProveS study please click here.
Odiparcil in Mucopolysaccharidosis Type VI (MPS VI)
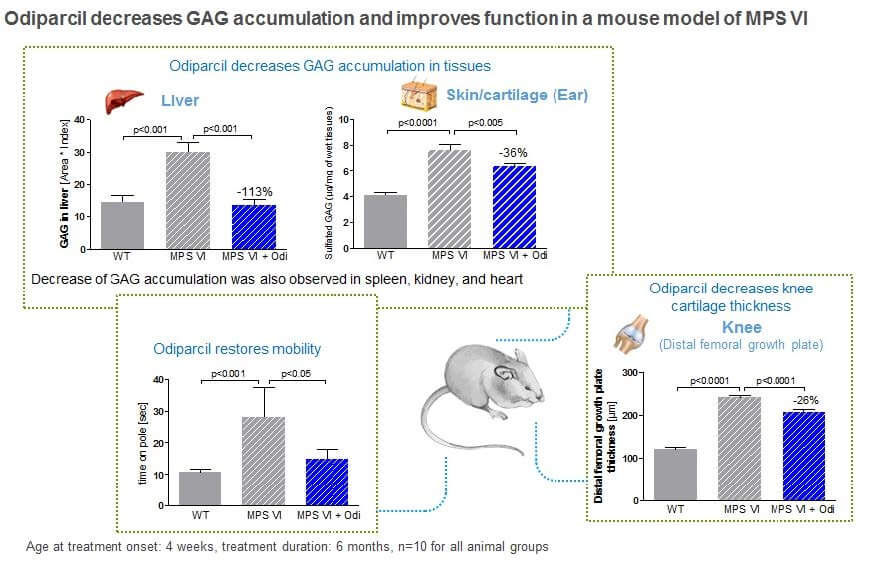
In arylsulfatase B mutant mice, a relevant model for mucopolysaccharidosis type VI (MPS VI), administration of odiparcil was associated with reductions of GAGs levels in tissues and organs relevant to the human disease.
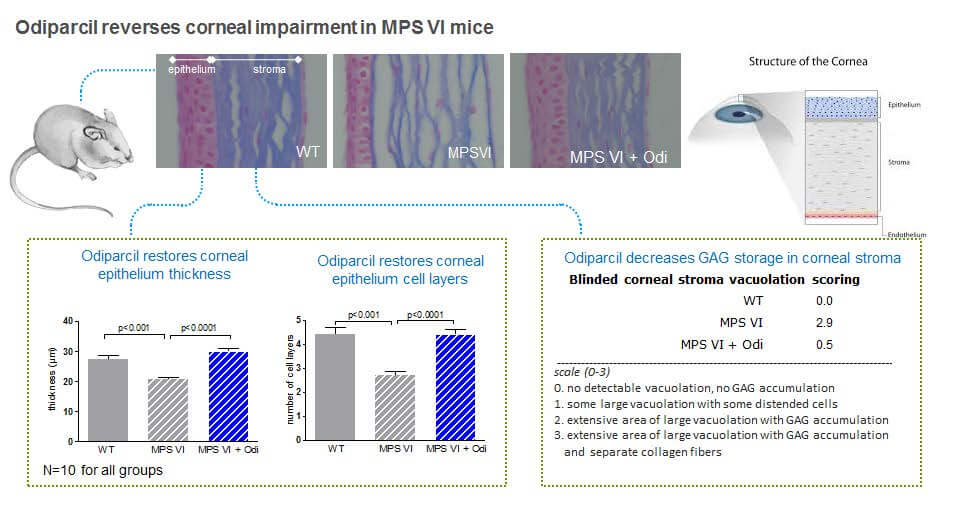
In this same MPS VI mouse model, we also observed that odiparcil was active in cartilage tissues. As shown in the figures below, we observed that treatment of MPS VI mice with odiparcil was associated with a decrease in thickness of the trachea and knee cartilage. In addition, we observed statistically significant improvements in mobility in the MPS VI mice treated with odiparcil. In this same MPS VI mouse model, we also observed that odiparcil was active in eye tissues.
As shown in the figures below, we observed that corneal thickness and the number of cell layers in the corneal epithelium were decreased in MPS VI mice treated with odiparcil. We also tested the accumulation of GAGs in the corneal stroma, which is a layer of the cornea in which GAGs are known to accumulate in MPS patients and observed decreases of GAGs accumulation in MPS VI mice treated with odiparcil in comparison to untreated MPS VI mice.
We observed that administration of odiparcil was associated with a decrease of CS intracellular content, while increasing the extra cellular level of GAGs.
Odiparcil clinical safety data
In clinical trials conducted in other indications prior to our founding, odiparcil was administered to over 1,800 subjects.
In these trials, odiparcil displayed a favorable safety and tolerability profile at daily doses in excess of the therapeutic range. Low toxicity was also observed in in vivo toxicology studies of up to 36 weeks.
The iMProveS trial confirmed this favourable safety profile in MPS VI patients.


