We are developing our lead product candidate, lanifibranor, for the treatment of patients with non-alcoholic steatohepatitis, or NASH.
Lanifibranor is an orally-available small molecule that acts to induce anti-fibrotic, anti-inflammatory as well as beneficial metabolic changes in the body by activating each of the three PPAR isoforms, known as PPARα, PPARδ and PPARɣ.
PPARs are ligand-activated transcription factors belonging to the nuclear hormone receptor family that regulate the expression of genes. PPARs play essential roles in the regulation of cellular differentiation, development and tumorigenesis.
Lanifibranor is a PPAR agonist that is designed to target all three PPAR isoforms in a moderately potent manner, with a well-balanced activation of PPARα and PPARδ, and a partial activation of PPARɣ. While there are other PPAR agonists that target only one or two PPAR isoforms for activation, lanifibranor is the only pan-PPAR agonist in clinical development. For an extensive discussion on the benefit of PPAR agonism in NASH please visit the panNASH Initiative website.
Lanifibranor
in Non-Alcoholic Steatohepatitis (NASH)
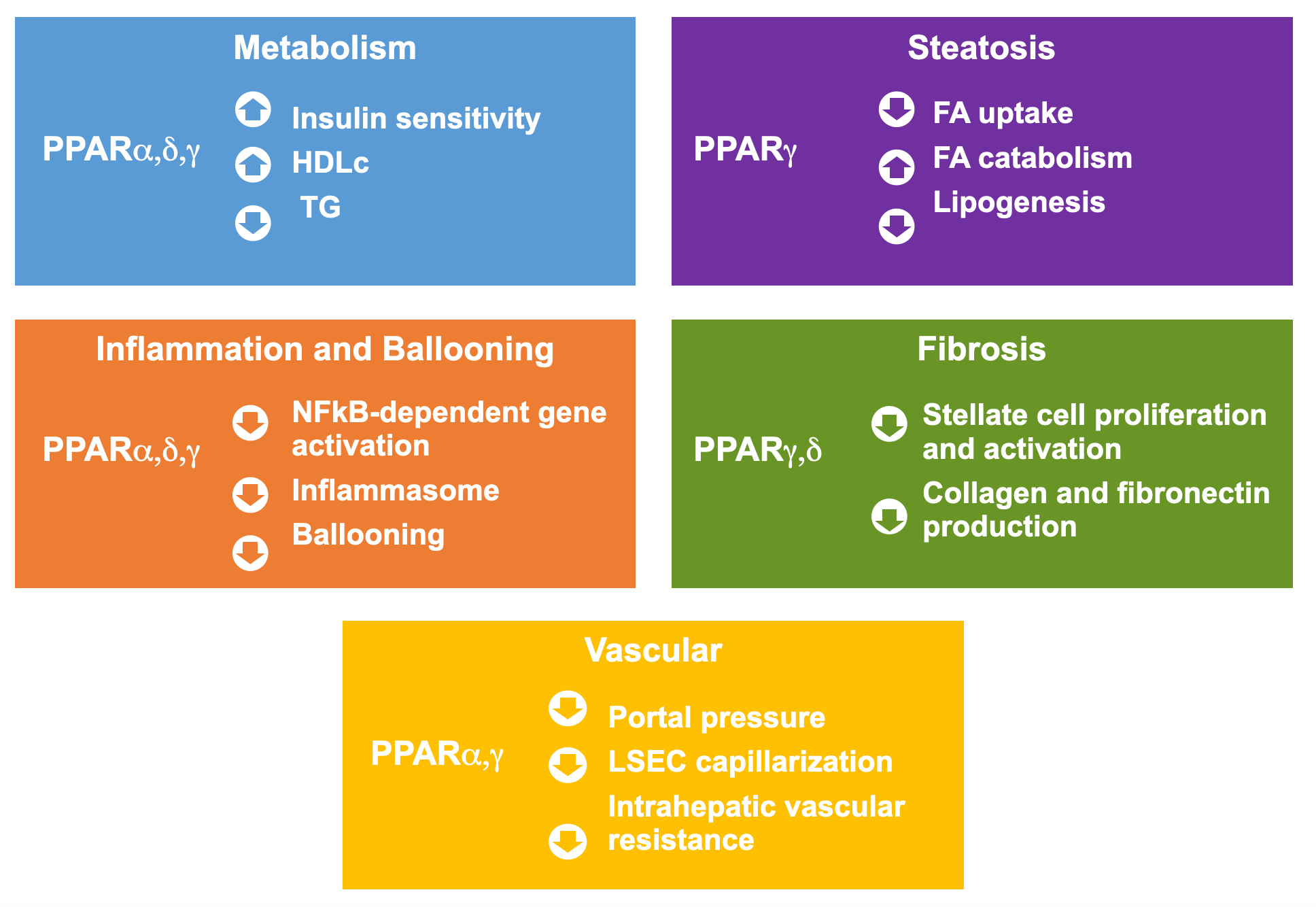
PPAR activation has been established to play a role in regulating each of the four components of NASH:
- Metabolism: Activation of PPARα and PPARδ has been demonstrated to reduce triglyceride levels and increase HDL cholesterol levels, while activation of PPARɣ has been demonstrated to increase insulin sensitization, all of which are key metabolic markers in patients with NASH.
- Steatosis: Activation of PPARα and PPARɣ addresses key elements of steatosis by enhancing fatty acid metabolism and ultimately decreasing lipogenesis.
- Inflammation and ballooning: Activation of PPARα, PPARδ and PPARɣ has been associated with statistically significant reductions of inflammation and ballooning.
- Fibrosis: Activation of PPARɣ is associated with anti-fibrotic effects across the process of fibrosis, from the production of stellate cells to the production of fibrotic proteins such as collagen and fibronectin.
In addition, lanifibranor has shown clear beneficial effects in a pre-clinical model of decompensated cirrhosis, which leads to a marked improvement in fibrosis and portal hypertension.
Based on the established role that PPAR activation plays in regulating metabolic processes, including steatosis, as well as in inflammatory and fibrotic processes, we are developing lanifibranor for the treatment of NASH.
Lanifibranor is the only product candidate in clinical development targeting all three PPAR isoforms. We believe that this pan-PPAR approach provides for a combination of anti-fibrotic, anti-inflammatory and beneficial metabolic effects that cannot be obtained with single and dual PPAR agonists.