We develop novel and differentiated oral small molecule therapies for patients suffering from diseases with significant unmet medical need
Our goal is to rapidly deliver multiple, novel and differentiated oral small molecule therapies to patients suffering from diseases with significant unmet medical need. We are focused on the areas of fibrosis, lysosomal storage disorders and oncology.
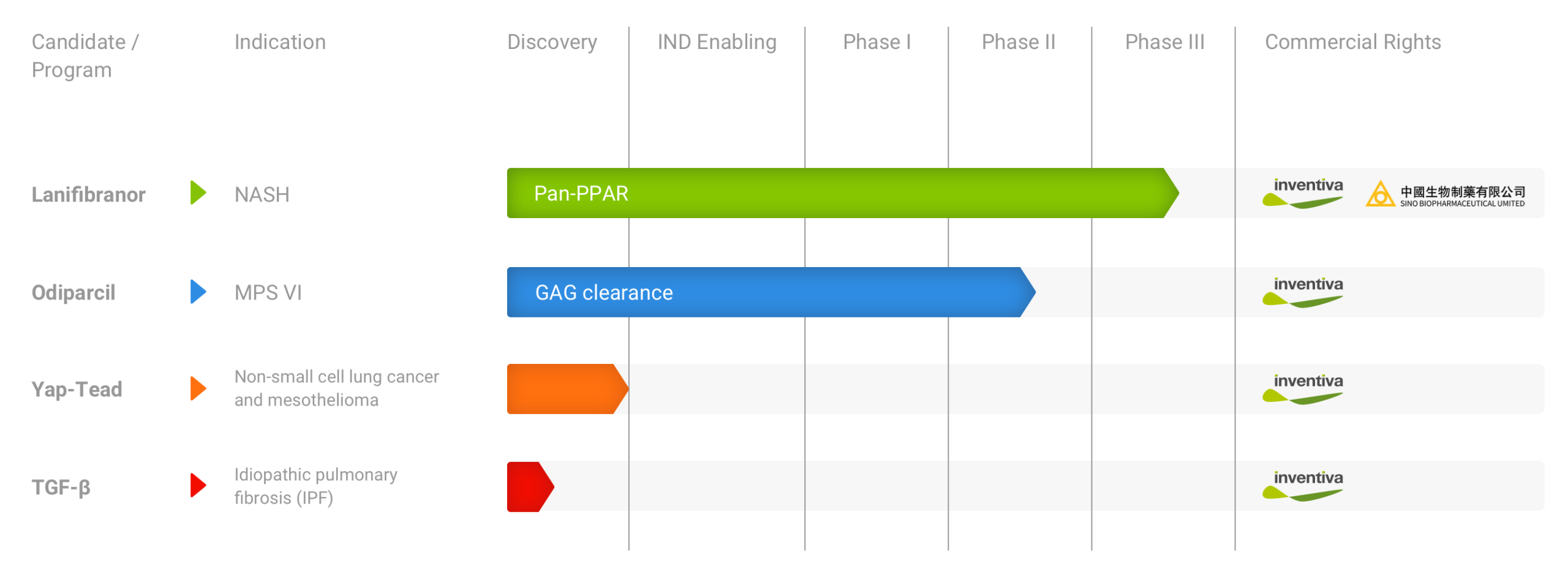
We are developing our lead product candidate, lanifibranor, for the treatment of patients with non-alcoholic steatohepatitis, or NASH. Lanifibranor is an orally-available small molecule that acts to induce anti-fibrotic, anti-inflammatory and beneficial metabolic changes in the body by activating all three peroxisome proliferator-activated receptor, or PPAR, isoforms.
Our second clinical-stage asset is odiparcil, which we are developing for the treatment of patients with mucopolysaccharidoses, or MPS, a group of rare genetic disorders characterized by an excessive accumulation of large sugar chains, known as glycosaminoglycans, or GAGs, in cells. Odiparcil is an orally-available small molecule designed to modify how GAGs are synthesized. Odiparcil acts to facilitate the production of soluble GAGs that can be excreted in the urine, rather than accumulating in cells.
Our Pipeline